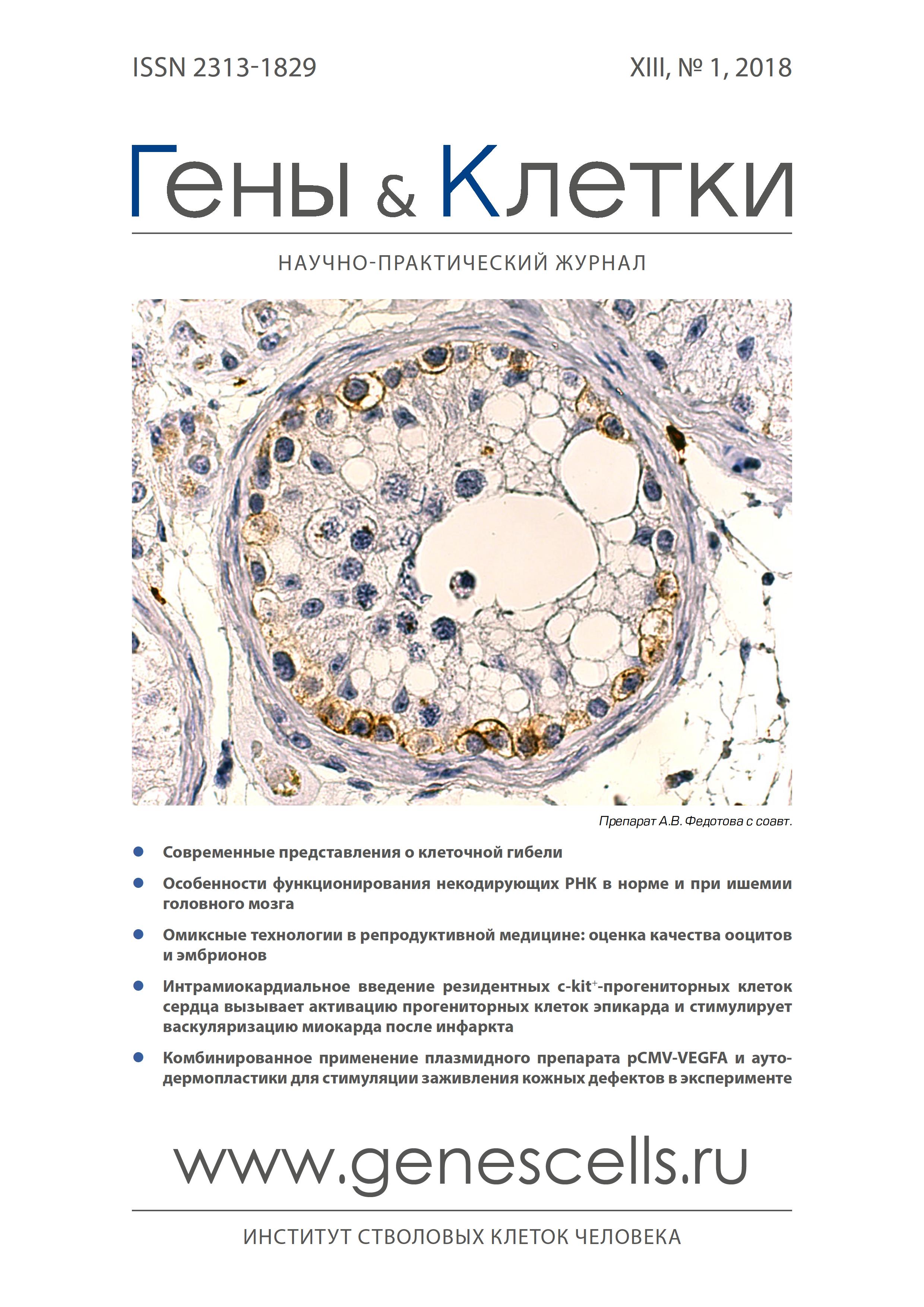
Intramiocardial administration of resident c-kit+ cardiac progenital cells activates epicardial progenitor cells and promotes myocardial vascularation after the infarction
- Authors: Dergilev K.V1, Tsokolaeva Z.I1, Beloglazova I.B1,2, Zubkova E.S1,2, Boldyreva M.A1,2, Ratner E.I1, Diykanov D.T2, Menshikov M.U1, Parfenova E.V1,2
-
Affiliations:
- Russian Cardiology Research and Production Complex
- M.V. Lomonosov Moscow State University
- Issue: Vol 13, No 1 (2018)
- Pages: 75-81
- Section: Articles
- Submitted: 05.01.2023
- Published: 15.03.2018
- URL: https://genescells.ru/2313-1829/article/view/120736
- DOI: https://doi.org/10.23868/201805009
- ID: 120736
Cite item
Abstract
Full Text
About the authors
K. V Dergilev
Russian Cardiology Research and Production Complex
Email: doctorkote@gmail.com
Z. I Tsokolaeva
Russian Cardiology Research and Production Complex
I. B Beloglazova
Russian Cardiology Research and Production Complex; M.V. Lomonosov Moscow State University
E. S Zubkova
Russian Cardiology Research and Production Complex; M.V. Lomonosov Moscow State University
M. A Boldyreva
Russian Cardiology Research and Production Complex; M.V. Lomonosov Moscow State University
E. I Ratner
Russian Cardiology Research and Production Complex
D. T Diykanov
M.V. Lomonosov Moscow State University
M. U Menshikov
Russian Cardiology Research and Production Complex
E. V Parfenova
Russian Cardiology Research and Production Complex; M.V. Lomonosov Moscow State University
References
- Cochain C., Channon K.M., Silvestre J.S. Angiogenesis in the infarcted myocardium. Antioxid. Redox Signal. 2013; 18(9): 1100-13.
- Shah A.M., Mann D.L. In search of new therapeutic targets and strategies for heart failure: recent advances in basic science. Lancet 2011; 378(9792): 704-12.
- Battegay E.J. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J. Mol. Med. 1995; 73(7): 333-46.
- Meoli D.F., Sadeghi M.M., Krassilnikova S. et al. Noninvasive imaging of myocardial angiogenesis following experimental myocardial infarction. J. Clin. Invest. 2004; 113(12): 1684-91.
- Uchida Y., Yanagisawa-Miwa A., Nakamura F. et al. Angiogenic therapy of acute myocardial infarction by intrapericardial injection of basic fibroblast growth factor and heparin sulfate: an experimental study. Am. Heart J. 1995; 130(6): 1182-8.
- Gyöngyösi M., Wojakowski W., Lemarchand P. et al. Meta-analysis of cell-based CaRdiac stUdiEs (ACCRUE) in patients with acute myocardial infarction based on individual patient data. Circ. Res. 2015; 116(8): 1346-60.
- Scimia M.C., Gumpert A.M., Koch W.J. Cardiovascular gene therapy for myocardial infarction. Expert Opin. Biol. Ther. 2014; 14(2): 183-95.
- Bearzi C., Rota M., Hosoda T. et al. Human cardiac stem cells. PNAS USA 2007; 104(35): 14068-73.
- Bolli R., Chugh A.R., D'Amario D. et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 2011; 378(9806): 1847-57.
- Fazel S., Cimini M., Chen L. et al. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J. Clin. Invest. 2006; 116(7): 1865-77.
- Kajstura J., Urbanek K., Perl S. et al. Cardiomyogenesis in the adult human heart. Circ. Res. 2010; 107(2): 305-15.
- Dergilev K.V., Makarevich P.I., Tsokolaeva Z.I. et al. Comparison of cardiac stem cell sheets detached by Versene solution and from thermo-responsive dishes reveals similar properties of constructs. Tissue Cell 2017; 49(1): 64-71.
- Traktuev D.O., Tsokolaeva Z.I., Shevelev A.A. et al. Urokinase gene transfer augments angiogenesis in ischemic skeletal and myocardial muscle. Mol. Ther. 2007; 15(11): 1939-46.
- Hochman J.S., Choo H. Limitation of myocardial infarct expansion by reperfusion independent of myocardial salvage. Circulation 1987; 75(1): 299-306.
- Asahara T., Murohara T., Sullivan A. et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275(5302): 964-7.
- Lin C.S., Lue T.F. Defining vascular stem cells. Stem Cells Dev. 2013; 22(7): 1018-26.
- Meizlish J.L., Berger H.J., Plankey M. et al.Functional left ventricular aneurysm formation after acute anterior transmural myocardial infarction. Incidence, natural history, and prognostic implications. N. Engl. J. Med. 1984; 311(16): 1001-6.
- Xiao Y., Ding L., Chen H. et al. Grain-Moxibustion may Protect Myocardium by Reducing Oxidative Stress in Doxorubicin-induced Cardiomyopathy Rats. Zhen Ci Yan Jiu. 2016; 41(6): 502-8.
- Paul A., Mitra A., Kohli V. et al. Anaesthetic challenges for device closure of post-infarct ventricular septal defect with coronary angioplasty. Ann. Card. Anaesth. 2003; 6(1): 52-5.
- Duim S.N., Smits A.M., Kruithof B.P. et al. The roadmap of WT1 protein expression in the human fetal heart. J. Mol. Cell. Cardiol. 2016; 90: 139-45.
- Duim S.N., Kurakula K., Goumans M.J. et al. Cardiac endothelial cells express Wilms' tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol. 2015; 81: 127-35.
- Xiang F.L., Liu Y., Lu X. et al. Cardiac-specific overexpression of human stem cell factor promotes epicardial activation and arteriogenesis after myocardial infarction. Circ. Heart Fail. 2014; 7(5): 831-42.
- Carmeliet P., Jain R.K. Angiogenesis in health and disease. Nat. Med. 2003; 9: 653-60.
- He L., Huang X., Kanisicak O. et al. Preexisting endothelial cells mediate cardiac neovascularization after injury. J. Clin. Invest. 2017; 127(8): 2968-81.
- Gômez-Gaviro M.V., Lovell-Badge R., Fernandez-Avilés F. et al. The vascular stem cell niche. J. Cardiovasc. Transl. Res. 2012; 5(5): 618-30.
- Bearzi C., Leri A., Lo Monaco F. et al. Identifikation of a coronary vascular progenitor cell in the human heart. PNAS USA 2009; 106(37): 15885-90.
- Hamdi H., Furuta A., Bellamy V. et al. Cell delivery: intramyocardial injections or epicardial deposition? A head-to-head comparison. Ann. Thorac. Surg. 2009; 87(4): 1196-203.
- Bogers A.J., Gittenberger-de Groot A.C., Poelmann R.E. et al. Development of the origin of the coronary arteries, a matter of ingrowth or outgrowth? Anat. Embryol. (Berl.) 1989; 180(5): 437-41.
- Reese D.E., Mikawa T., Bader D.M. Development of the coronary vessel system. Circ. Res. 2002; 91(9): 761-8.
- Van den Akker N.M., Winkel L.C., Nisancioglu M.H. et al. PDGF-B signaling is important for murine cardiac development: its role in developing atrioventricular valves, coronaries, and cardiac innervation. Dev. Dyn. 2008; 237(2): 494-503.
- Tang Y., Wu X., Lei W. et al. TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat. Med. 2009; 15(7): 757-65.
- Chimenti I., Smith R.R., Li T.S. et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 2010; 106: 971-80.
- Tomanek R.J., Ratajska A., Kitten G.T. et al. Vascular endothelial growth factor expression coincides with coronary vasculogenesis and angiogenesis. Dev. Dyn. 1999; 215(1): 54-61.
- Folkman J., D'Amore P.A. Blood vessel formation: what is its molecular basis? Cell 1996; 87(7): 1153-5.
- Kaminski W.E., Lindahl P., Lin N.L. et al. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood 2001; 97(7): 1990-8.
- Austin A.F., Compton L.A., Love J.D. et al. Primary and immortalized mouse epicardial cells undergo differentiation in response to TGFbeta. Dev. Dyn. 2008; 237(2): 366-76.
- Eisenberg L.M., Markwald R.R. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ. Res. 1995; 77(1): 1-6.
- Vrijsen K.R., Sluijter J.P., Schuchardt M.W. et al. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J. Cell. Mol. Med. 2010; 14: 1064-70.
Supplementary files