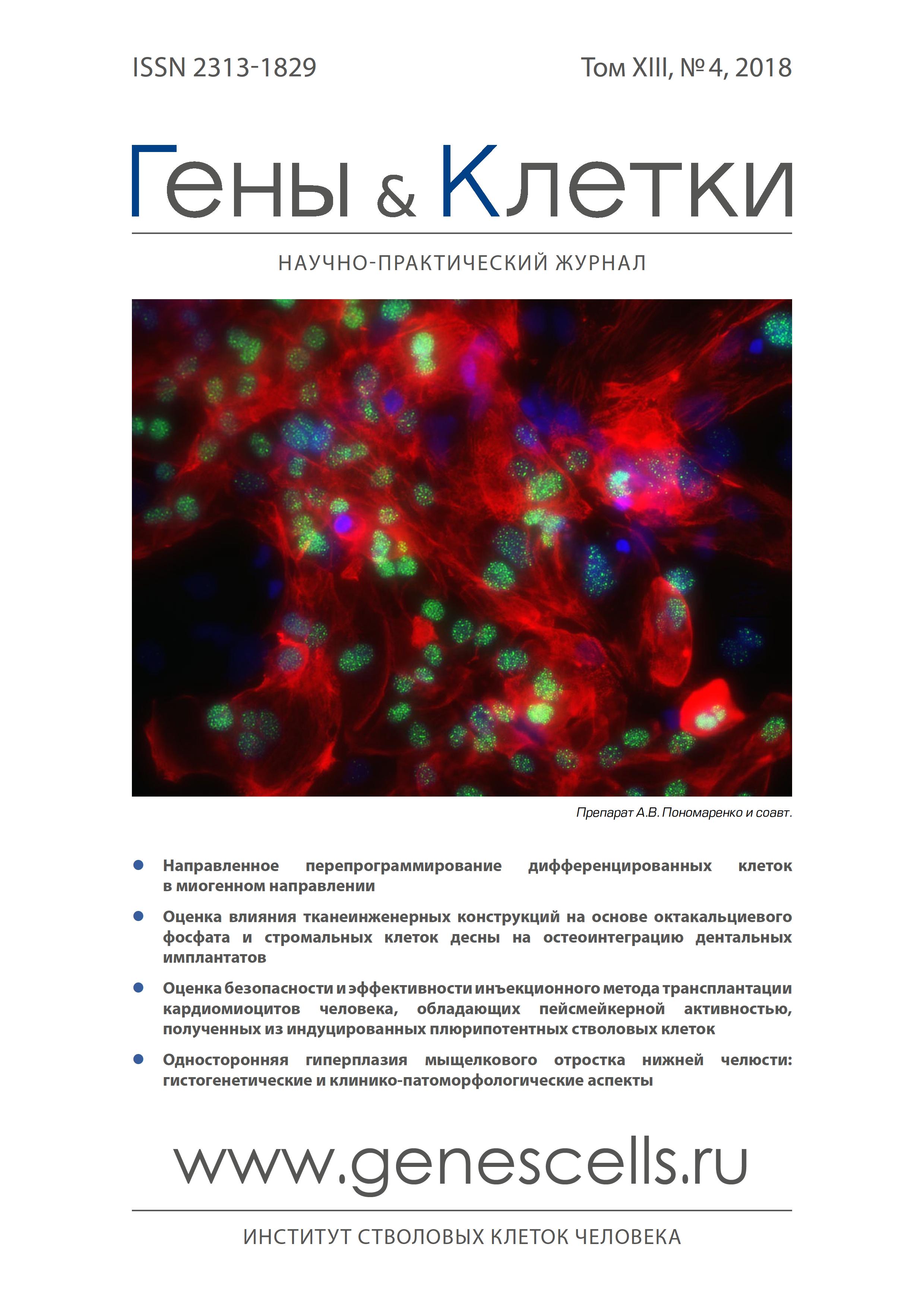
Survival and functional activity examination of cardiomyocytes differentiated from human iPSCs, when transplanting in SCID mice
- Authors: Pavlova S.V1,2,3, Chepeleva E.V2, Dementyeva E.V1,2,3, Grigor'eva E.V1,2,3,4, Sorokoumov E.D5, Slotvitsky M.M6, Ponomarenko A.V2, Dokuchaeva A.A2, Malakhova A.A1,2,3,4, Sergeevichev D.S2, Pokushalov E.A2, Zakian S.M1,2,3,4
-
Affiliations:
- Federal Research Center Institute of Cytology and Genetics of the SO of the RAS
- E.N. Meshalkin National Medical Research Center
- Institute of Chemical Biology and Fundamental Medicine of the SO of the RAS
- Novosibirsk State University
- Institute of Computational Technologies of the SO of the RAS
- Moscow Institute of Physics and Technology (State University)
- Issue: Vol 13, No 4 (2018)
- Pages: 51-60
- Section: Articles
- URL: https://genescells.ru/2313-1829/article/view/120735
- DOI: https://doi.org/10.23868/201812047
- ID: 120735
Cite item
Abstract
Full Text
About the authors
S. V Pavlova
Federal Research Center Institute of Cytology and Genetics of the SO of the RAS; E.N. Meshalkin National Medical Research Center; Institute of Chemical Biology and Fundamental Medicine of the SO of the RAS
Email: sonpavlova@gmail.com
E. V Chepeleva
E.N. Meshalkin National Medical Research Center
E. V Dementyeva
Federal Research Center Institute of Cytology and Genetics of the SO of the RAS; E.N. Meshalkin National Medical Research Center; Institute of Chemical Biology and Fundamental Medicine of the SO of the RAS
E. V Grigor'eva
Federal Research Center Institute of Cytology and Genetics of the SO of the RAS; E.N. Meshalkin National Medical Research Center; Institute of Chemical Biology and Fundamental Medicine of the SO of the RAS; Novosibirsk State University
E. D Sorokoumov
Institute of Computational Technologies of the SO of the RAS
M. M Slotvitsky
Moscow Institute of Physics and Technology (State University)
A. V Ponomarenko
E.N. Meshalkin National Medical Research Center
A. A Dokuchaeva
E.N. Meshalkin National Medical Research Center
A. A Malakhova
Federal Research Center Institute of Cytology and Genetics of the SO of the RAS; E.N. Meshalkin National Medical Research Center; Institute of Chemical Biology and Fundamental Medicine of the SO of the RAS; Novosibirsk State University
D. S Sergeevichev
E.N. Meshalkin National Medical Research Center
E. A Pokushalov
E.N. Meshalkin National Medical Research Center
S. M Zakian
Federal Research Center Institute of Cytology and Genetics of the SO of the RAS; E.N. Meshalkin National Medical Research Center; Institute of Chemical Biology and Fundamental Medicine of the SO of the RAS; Novosibirsk State University
References
- Burridge P.W., Matsa E., Shukla P. et al. Chemically defined generation of human cardiomyocytes. Nat. Methods 2014; 11(8): 855-60.
- Lian X., Zhang J., Azarin S.M. et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/ß-catenin signaling under fully defined conditions. Nat. Protoc. 2013; 8(1): 162-75.
- Agladze N.N., Halaidych O.V., Tsvelaya V.A. et al. Synchronization of excitable cardiac cultures of different origin. Biomater. Sci. 2017; 5: 1777-85.
- Shadrin I.Y., Allen B.W., Qian Y. et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 2017; 8(1): 1825-40.
- Masuda S., Shimizu T. Three-dimensional cardiac tissue fabrication based on cell sheet technology. Adv. Drug Deliv. Rev. 2016; 96: 103-9.
- Funakoshi S., Miki K., Takaki T. et al. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci. Rep. 2016; 6: 1-14.
- Riegler J., Tiburcy M., Ebert A. et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015; 117(8): 720-30.
- Matsuo T., Masumoto H., Tajima S. et al. Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells. Sci. Rep. 2015; 5: 1-15
- Chong J.J.H., Yang X., Don C.W. et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014; 510(7504): 273-7.
- Yaniv Y., Spurgeon H.A., Lyashkov A.E. et al. Crosstalk between mitochondrial and sarcoplasmic reticulum Ca2+ cycling modulates cardiac pacemaker cell automaticity. PLoS One 2012; 7(5): 1-13.
- Zhang X.H., Wei H., Saric T. et al. Regionally diverse mitochondrial calcium signaling regulates spontaneous pacing in developing cardiomyocytes. Cell Calcium 2015; 57(5-6): 321-36.
- Morad M., Zhang X. Mechanisms of spontaneous pacing: SA-nodal cells, neonatal cardiomyocytes, and human Stem cell derived cardiomyocytes. Can. J. Physiol. Pharmacol. 2017; 95(10): 1100-7.
- Байрамова С.А., Стрельников А.Г., Романов А.Б. и др. Перспективы создания пейсмейкерной сердечной ткани с использованием современных генетических и тканеинженерных технологий. Гены и Клетки 2017; XII(2): 29-36.
- Grigor'eva E.V., Malankhanova T.B., Surumbayeva A. et al. Generation and characterization of iPSCs from human embryonic dermal fibroblasts of a healthy donor from Siberian population. BioRxiv https://doi. org/10.1101/455535.
- Слотвицкий М.М., Цвелая В.А., Фролова Ш.Р. и др. Исследование функциональности получаемых из индуцированных плюрипотентных стволовых клеток кардиомиоцитов для моделирования сердечных аритмий при синдроме удлиненного интервала QT. Вавиловский журнал генетики и селекции 2018; 22(2): 187-95.
- Gao E., Lei Y.H., Shang X. et al. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ. Res. 2010; 107(12): 1445-53.
- Чепелева Е.В., Балашов В.А., Докучаева А.А. и др. Исследование биологической совместимости полилактидных нановолоконных матриксов, заселенных кардиальной клеточной культурой, в эксперименте на мини-свиньях. Гены и Клетки 2017; XII(4): 62-8.
- Чепелева Е.В., Павлова С.В., Малахова А.А. и др. Терапия хронического кардиосклероза у крыс линии WAG культурами кардиоваскулярных клеток, обогащенными стволовыми клетками сердца. Клеточные технологии в биологии и медицине 2015; 3: 56-65.
- Dubois N.C., Craft A.M., Sharma P. et al. SIRPA is a specific cell-surface marker for isolating cardiomyocytes derived from human pluripotent stem cells. Nat. Biotechnol. 2011; 29(11): 1-13.
- Ye W., Wang J., Song Y. et al. A common Shox2-Nkx2.5 antagonistic mechanism primes the pacemaking cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015; 142: 2521-32.
- van Weerd J.H., Christoffels V.M. The formation and function of the cardiac conduction system. Dev. 2016; 143(2): 197-210.
- Protze S.I., Liu J., Nussinovitch U. et al. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2016; 12: 1-16.
- Kermani P., Rafii S., Hempstead B.L. et al. Neurotrophins promote revascularization by local recruitment of TrkB + endothelial cells and systemic mobilization of hematopoietic progenitors. The Journal of Clinical Investigation 2005; 115(3):653-63.
- Zakharova I.S., Zhiven’ M.K., Saaya S.B. et al. Endothelial and smooth muscle cells derived from human cardiac explants demonstrate angiogenic potential and suitable for design of cell-containing vascular grafts. J. Transl. Med. 2017; 15(1): 54-70.
- Kolanowski T.J., Antos C.L., Guan K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. International Journal of Cardiology 2017; 241: 379-86.
- Duelen R., Sampaolesi M. Stem cell technology in cardiac regeneration: A Pluripotent Stem Cell Promise. EBioMedicine 2017; 16: 30-40.
- Ronaldson-Bouchard K., Ma S.P., Yeager K. et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018; 556: 239-43.
- Eng G., Lee B.W., Protas L. et al. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat. Commun. 2016; 7: 1-10.
- Li J., Minami I., Shiozaki M. et al. Human pluripotent stem cell-derived cardiac tissue-like constructs for repairing the infarcted myocardium. Stem Cell Reports 2017; 9(5): 1546-59.
- Liu Y., Chen B., Yang X. et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018; 36(7): 597-605.
- Chauveau S., Anyukhovsky E.P., Ben-Ari M. et al. Induced pluripotent stem cell-derived cardiomyocytes provide in vivo biological pacemaker function. Circ. Arrhythmia Electrophysiol. 2017; 10: 1-10.
- Павлова С.В., Перовский П.П., Чепелева Е.В. и др. Характеристика кардиальных культур клеток, полученных из экспланта сердечной мышцы человека. Клеточные технологии в биологии и медицине 2013; 3: 132-41.
- Павлова С.В., Сергеевичев Д.С., Чепелева Е.В. и др. Сравнение мезенхимальных стромальных клеток костного мозга и региональных стволовых клеток сердца и фибробластов кожи человека. Патология кровообращения и кардиохирургия 2015; 19(4-2): 12-9.
- Павлова С.В., Розанова И.А., Чепелева Е.В. и др. Ангиогенный потенциал кардиальных стволовых и мезенхимальных стромальных клеток костного мозга крысы. Патология кровообращения и кардиохирургия 2015; 19(4-2): 77-84.
- Павлова С.В., Леонова Е.А., Чепелева Е.В. и др. Мониторинг трансплантации клеток кардиосфер в фибриновом геле в зону ишемического повреждения миокарда с использованием люциферазной репортерной системы. Гены и Клетки 2017; XII(4): 69-75.
Supplementary files