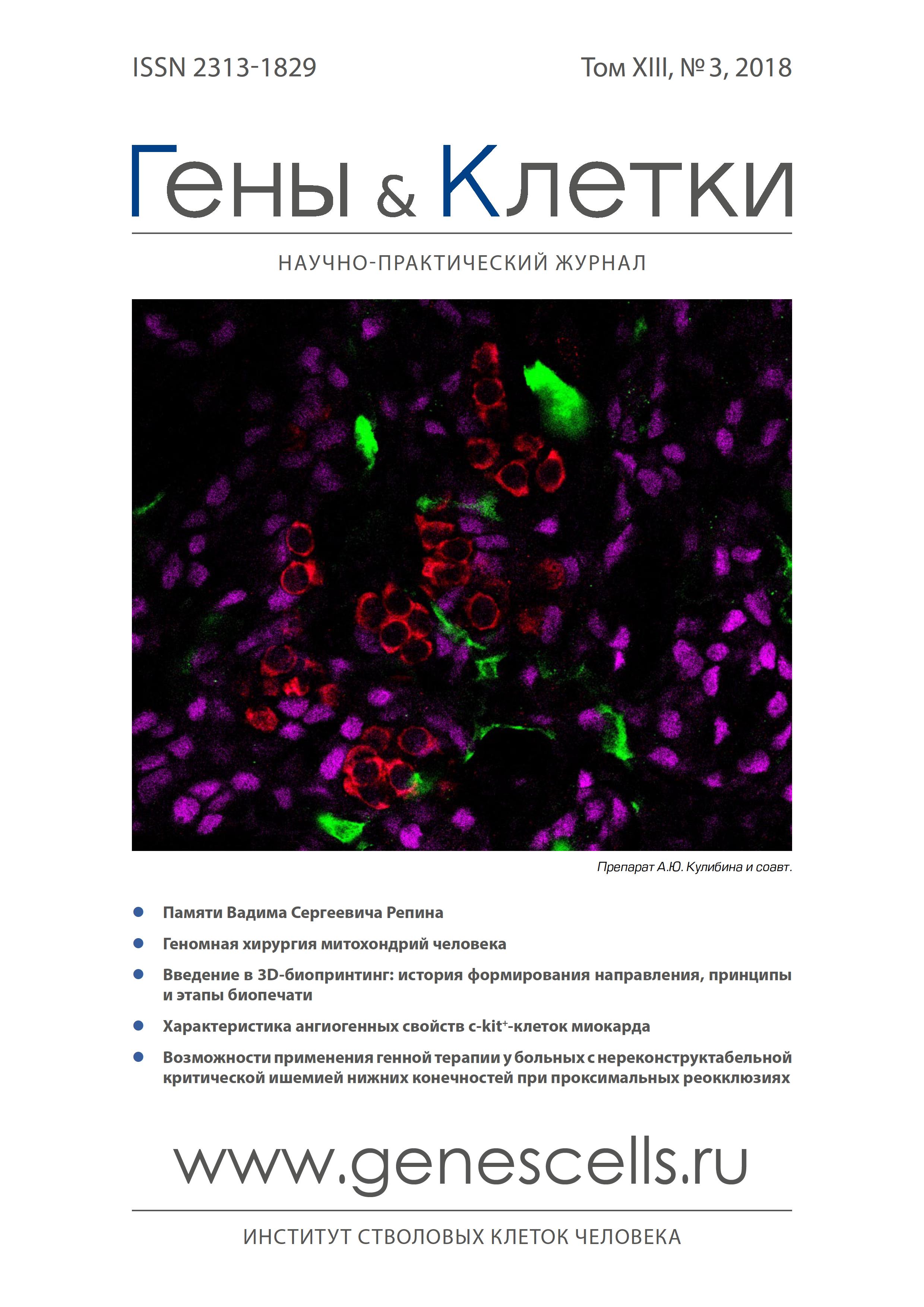
The change in the quantity of macrophages and their stabilin-1 + M2 subpopulation in the myocardium in patients during early postinfarction period
- Authors: Rebenkova M.S1,2, Gombozhapova A.E1,2, Rogovskaya Y.V1,2, Ryabov V.V1,2,3, Churina E.G2,3, Kzhyshkowska J.G2,4
-
Affiliations:
- Cardiology Research Institute, Tomsk National Research Medical Center of the RAS
- National Research Tomsk State University
- Siberian State Medical University
- Ruprecht-Karls University of Heidelberg
- Issue: Vol 13, No 3 (2018)
- Pages: 56-62
- Section: Articles
- URL: https://genescells.ru/2313-1829/article/view/120734
- DOI: https://doi.org/10.23868/201811034
- ID: 120734
Cite item
Abstract
Keywords
Full Text
About the authors
M. S Rebenkova
Cardiology Research Institute, Tomsk National Research Medical Center of the RAS; National Research Tomsk State University
A. E Gombozhapova
Cardiology Research Institute, Tomsk National Research Medical Center of the RAS; National Research Tomsk State University
Y. V Rogovskaya
Cardiology Research Institute, Tomsk National Research Medical Center of the RAS; National Research Tomsk State University
VV. V Ryabov
Cardiology Research Institute, Tomsk National Research Medical Center of the RAS; National Research Tomsk State University; Siberian State Medical University
EG. G Churina
National Research Tomsk State University; Siberian State Medical University
J. G Kzhyshkowska
National Research Tomsk State University; Ruprecht-Karls University of Heidelberg
References
- Montecucco F., Carbone F., Schindler T.H. Pathophysiology of ST-segment elevation myocardial infarction: novel mechanisms and treatment. Eur. Heart J. 2016; 37: 1268-83.
- Rafatian N., Westcott K.V., White R.A. et al. Cardiac macrophages and apoptosis after myocardial infarction: effects of central MR blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014; 307[7]: 79-87.
- Рябов В.В., Гомбожапова А.Э., Роговская Ю.В. и др. Функциональная пластичность моноцитов/макрофагов в процессах восстановительной регенерации и постинфарктного ремоделирования сердца. Иммунология 2016; 37[6]: 305-12.
- Ismahil M.A., Hamid T., Bansal S.S. et al. Remodeling of the mononuclear phagocyte network underlies chronic inflammation and disease progression in heart failure: critical importance of the cardiosplenic axis. Circ. Res. 2014; 114[2]: 266-82.
- de Couto G., Liu W., Tseliou E. et al. Macrophages mediate cardio-protective cellular postconditioning in acute myocardial infarction. J. Clin. Invest. 2015; 125[8]: 3147-62.
- Troidl C., Mollmann H., Nef H. et al. Classically and alternatively activated macrophages contribute to tissue remodelling after myocardial infarction. J. Cell. Mol. Med. 2009; 13: 3485-96.
- Gombozhapova A., Rogovskaya Y., Shurupov V. et al. Macrophage activation and polarization in post-infarction cardiac remodeling. Journal of Biomedical Science 2017; 24: 13.
- Ben-Mordechai T., Palevski D., Glucksam-Galnoy Y. et al. Targeting macrophage subsets for infarct repair. J. Cardiovasc. Pharmacol. Ther. 2015; 20[1]: 36-51.
- Nahrendorf M., Swirski F.K., Aikawa E. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 2007; 204: 3037-47.
- Tsujioka H., Imanishi T., Ikejima H. et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 2009; 54: 130-8.
- Zajac Е., Schweighofer B., Kupriyanova T.A. et al. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TiMp-1 complexed with their secreted proMMP-9. Blood 2013; 122[25]: 4054-67.
- Yu X., Xu M., Li N. et al. ß-elemene inhibits tumor-promoting effect of M2 macrophages in lung cancer. Biochem. Biophys. Res. Commun. 2017; 490[2]: 514-20.
- Chinetti-Gbaguidi G., Staels B. PPARbeta in macrophages and atherosclerosis. Biochimie 2017; 136: 59-64.
- Heidt T., Courties G., Dutta P. et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ. Res. 2014; 115[2]: 284-95.
- Gratchev A., Sobenin I., Orekhov A. et al. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology 2012; 5: 476-82.
- Kzhyshkowska J., Gudima A., Mogantia K. et al. Perspectives for monocyte/macrophage-based diagnostics of chronic inflammation. Transfus. Med. Hemother. 2016; 43: 66-77.
- Biswas S.K., Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat. Immunol. 2010; 11: 889-96.
- Murray P.J., Allen J.E., Biswas S.K. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41: 14-20.
- Schönhaar K., Schledzewski K., Michel J. et al. Expression of stabilin-1 in M2 macrophages in human granulomatous disease and melanocytic lesions. International journal of clinical and experimental pathology 2014; 7[4]: 1625-34.
- Kzhyshkowska J., Gudima A., Moganti K. et al. Perspectives for Monocyte/Macrophage-Based Diagnostics of Chronic Inflammation. Transfus. Med. Hemother. 2016; 43: 66-77.
- Palani S., Elima K., Ekholm E. et al. Monocyte Stabilin-1 suppresses the activation of Th1 lymphocytes. J. Immunol. 2016; 196[1]: 115-23.
- Rantakari P., Patten D.A., Valtonen J. et al. Stabilin-1 expression defines a subset of macrophages that mediate tissue homeostasis and prevent fibrosis in chronic liver injury. PNAS USA 2016; 113[33]: 9298-303.
- Kzhyshkowska J. Multifunctional receptor stabilin-1 in homeostasis and disease. Scientific World Journal 2010; 10: 2039-53.
- Miller C.M., Donner A.J., Blank E.E. et al. Stabilin-1 and Stabilin-2 are specific receptors for the cellular internalization of phosphorothioate-modified antisense oligonucleotides [ASOs] in the liver. Nucleic Acids Res. 2016; 44[6]: 2782-94.
- Гомбожапова А.Э., Роговская Ю.В., Ребенкова М.С. и др. CD68 и стабилин-1 позитивные макрофаги в постинфарктной регенерации миокарда. Российский кардиологический журнал 2017; 11[151]: 56-61.
- Gombozhapova A.E., Rogovskaya Y.V., Rebenkova M.S. et al. Myocardial stabilin-1-positive macrophages in patients with fatal myocardial infarction. The Siberian Medical Journal 2016; 31:100-3.
- Courties G., Heidt T., Sebas M. et al. In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J. Am. Coll. Cardiol. 2014; 63[15]: 1556-66.
Supplementary files